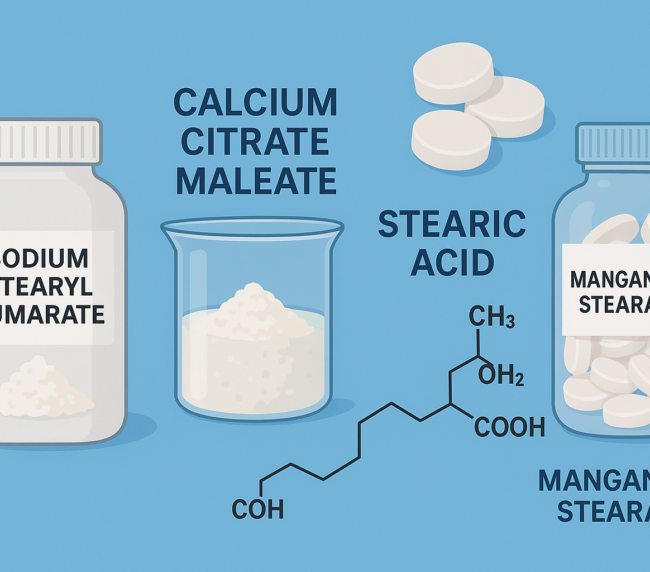
Zinc stearate is a fine, white, hydrophobic powder derived from stearic and palmitic acids. It is odorless or has a faint characteristic smell and is free from grittiness. It is insoluble in water, ethanol, and ether, but soluble in aromatic solvents and acids. Zinc stearate is primarily used as a lubricant in solid dosage forms and is also found in creams, ointments, and dusting powders. Its softening and melting range is 120–122 °C.
Chemical Name and CAS Registry Number :-Octadecanoic acid calcium salt [557-05-1]
Functional Category :-Tablet and capsule lubricant