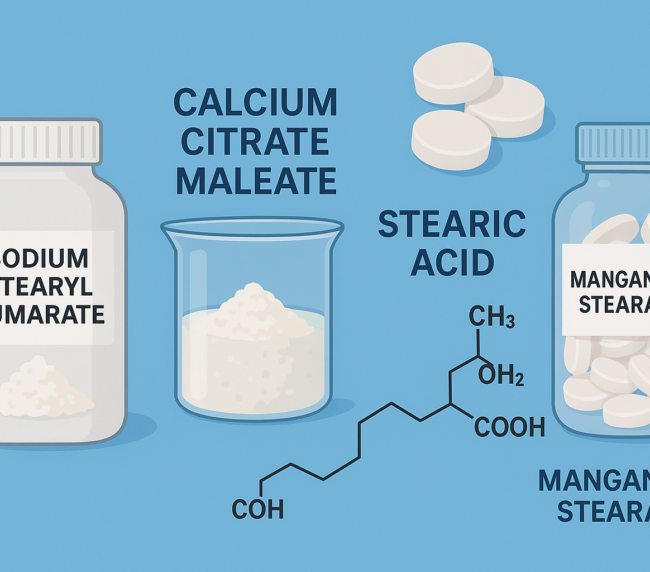
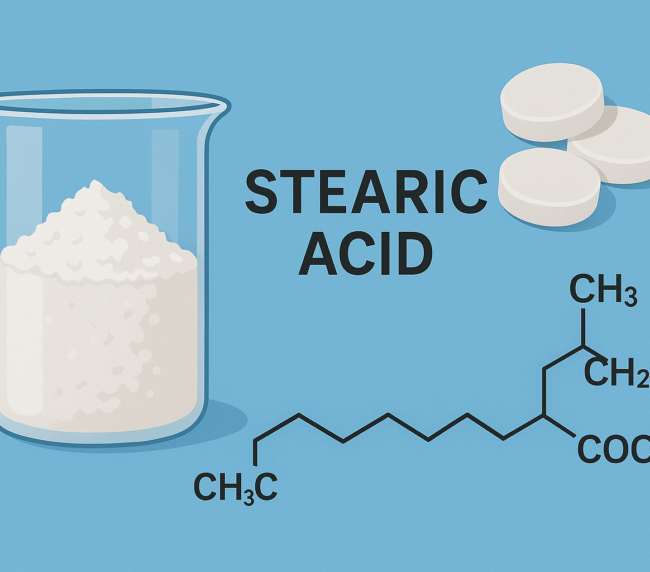
Sodium stearyl fumarate is a fine, white powder composed of agglomerates of flat, circular-shaped particles. It is practically insoluble in most organic solvents and water at room temperature, though solubility increases with temperature. It has a melting point of 224–245 °C (with decomposition), and a slightly alkaline pH of 8.3 in a 5% w/v aqueous solution at 90 °C. The bulk and tapped densities range from 0.2–0.5 g/cm³.
Chemical Name and CAS Registry Number :-2-Butenedioic acid, monooctadecyl ester, sodium salt [4070-80-8]
Functional Category :-Tablet and capsule lubricant.